
COVID-19 Surveillance Test Kit
Highlights
Stop the spread of Coronavirus with a simple, pooled, saliva-based test. Routinely test large groups such as classrooms, work teams, and other populations and detect infection within two hours.
- Rapid ResultsGo from sample to result in less than two hours.
- Gold Standard AccuracyCDC-recommended, RT-qPCR detection technology delivers precise results.
- Pain-Free Saliva CollectionMinimally invasive sample collection can be performed with no specialized training or materials.
- Easy-to-Use WorkflowSimple steps with software that walks the user through the testing process.
Description
COVID Surveillance
The COVID-19 Surveillance Test Kit enables rapid detection of COVID-19 in groups of people who interact regularly with one another. Results are used to inform health management actions.
If no virus is detected, daily school or workplace activities can be continued with care. Testing is an additional safety measure that provides peace-of-mind to students, parents, and employees.
If a virus is detected, take targeted action to stop the spread. Depending on the groups tested, results can be used to inform strategic individual classroom/departmental closures.
In the case of positive results, protect personnel by:
- Referring people for diagnostic testing
- Discontinuing group interactions
- Decontaminating classrooms or workspaces
Pooled Testing
Surveillance testing detects COVID-19 at the population level. The test kit incorporates a pooling step, to prevent obtaining a patient-specific result, which allows use outside of CLIA labs and healthcare settings.Reduce cost and increase testing capacity 5-fold
Compared to individual diagnostic testing, sample pooling conserves resources and can increase testing capacity by 2 – 5-fold.
Questions about surveillance or COVID-19 testing?
Talk to a SpecialistBenefits of Saliva
Saliva is the ideal specimen for routine testing. In addition to being a safe and easy-to-collect specimen for COVID testing, it’s:
- Pain-free and minimally invasive compared to deep nasopharyngeal swabbing
- Does not require specialized sample collection training
- Simple procedure reduces errors in sample collection, which can lead to unreliable results
- Invasive collection methods may induce sneezing and coughing, causing viral particles to be expelled. Viral exposure is minimized with a comfortable, self-collected procedure.
How It Works
Saliva Collection
Spit or drool at least 0.5 mL of saliva into the sterile specimen container and cap.
Pool and Extract
Pool saliva samples and prepare following step-by-step instructions. Up to 5 samples may be pooled per test. Add extraction buffer per the user manual.
Set Up RT-qPCR and Run
Prepare and aliquot mix into PCR tubes or plate. Add samples and controls. Load and run the qPCR instrument.
Get Results
Get clear results in 60 minutes. Software automatically analyzes control reactions to ensure quality.
View the user manual for the detailed protocol.
Accuracy Ensured
The Gold Standard of COVID Detection
The surveillance solution uses RT-qPCR, the detection method recommended by the CDC due to its high sensitivity and specificity. This approach makes exponential copies of the virus’s exact genetic material, allowing detection of even the smallest viral quantities.Detect an infection with as little as 5.8 viral particles/µL
In contrast, rapid antigen tests look for proteins on the virus’s surface and require thousands to tens of thousands of viral particles to detect an infection. Our test kit enables detection of an active infection with as little as 5.8 viral particles per microliter, which means you can discover COVID earlier.
Highly Controlled Reactions
A comprehensive control system ensures reaction performance and reliable results. The positive control verifies that the RT-qPCR reaction was set-up correctly, while the No Template Control confirms that the reaction is contamination-free. The endogenous control ensures that extraction and RT-qPCR was completed successfully, reducing the risk of false negatives. All controls and test kits are fully validated to the same standards as those used in diagnostic testing.
Reduce False Positives
The UNG carry-over prevention system minimizes false positives by reducing carryover contamination. The PCR mixture is treated with Uracil-DNA Glycosylase (UNG) which degrades leftover amplicons from previous runs before the next run begins.
Clear, Actionable Results
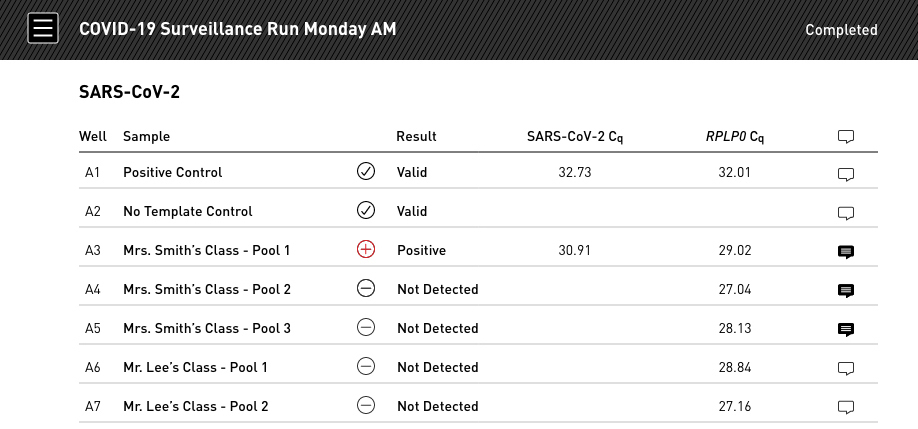
The Open qPCR’s software automates test execution and result analysis, making it an ideal choice for time-sensitive applications.
Results table is designed for interpretation by those from all technical levels, so you can proceed quickly to targeted next steps.
Quick Start Bundle
Chai’s COVID-19 Surveillance Quick Start Bundle
Contains everything you need to begin testing groups for Coronavirus:
Open qPCR instrument, dual channel
Protect your investment with comprehensive service plans:
- Covers all repairs for duration of plan
- Covers shipping for repairs within U.S.
- Covers "hot-swaps": if your unit requires service, we will send you a replacement device
COVID-19 Surveillance Test Kit for 100 tests
Equipment
Open qPCR instrument, dual channel
Protect your investment with comprehensive service plans:
- Covers all repairs for duration of plan
- Covers shipping for repairs within U.S.
- Covers "hot-swaps": if your unit requires service, we will send you a replacement device
Supplies
COVID-19 Surveillance Test Kit for 100 tests
Enzymatic DNA/RNA Extraction Buffer 10X
Filter tips for 10 μL, 100 μL and 1000 μL pipettes
Technical Specifications
| Limit of Detection | 5.8 viral copies/µL |
| Specimen Type | Saliva, unpreserved |
| Saliva Specimen Storage Conditions | Up to 16 hours at ambient Up to 72 hours at 4 ˚C |
| Reagent Storage Conditions | -20 ºC |
| Inclusivity | SARS-CoV-2 |
| Detection Channels | FAM, HEX |
| Endogenous Control | RPLP0 |
| Positive Control | Human RPLP0 and SARS-CoV-2 N gene fragments |
| UNG Carry-over Prevention | Included |
Kit Components
| Component | Amount |
| Sahara One-Step RT-qPCR Master Mix with UNG | 1.1 mL |
| COVID-19 Saliva Dx Oligo Mix | 415 µL |
| COVID-19 Saliva Dx Cofactor Buffer | 415 µL |
| COVID-19 Saliva Dx Positive Control | 110 µL |
| DNase/RNase-Free Distilled Water | 500 µL |
Questions about surveillance or COVID-19 testing?
Talk to a SpecialistFAQs
What is the difference between surveillance and diagnostic testing?
Diagnostic testing looks for occurrence of COVID-19 at the individual level when there is a reason to believe an individual may be infected. Surveillance testing looks for infection within a population or community, and can be used for making health management decisions at the population level.
For example, if routine COVID-19 surveillance reveals the presence of infection in a workplace population, management might refer individuals for diagnostic testing to determine who is infected, and could even elect to close a facility pending diagnostic test results to prevent further spread.
Can this kit be used for diagnostic testing?
No. Chai’s COVID-19 Surveillance Test Kit is marketed for surveillance and research use only. It is not for use in diagnostic procedures. Individual results may not be returned to the tested population. To prevent obtaining individual results in the first place, it is recommended to pool samples. Sample pooling also increases the capacity and speed of testing efforts and reduces costs.
For diagnostic testing, see our COVID-19 Saliva Dx Test Kit.
Is CLIA certification required to perform SARS-CoV-2 surveillance?
No. The US Centers for Medicare & Medicaid Services has stated that facilities using a pooled sampling procedure to report non patient-specific results do not require CLIA certification during the Public Health Emergency. The pooling feature of our COVID-19 Surveillance Test Kit is designed to allow facilities without CLIA certificates to meet this requirement.Can this assay detect Omicron, Delta, and other variants of SARS-CoV-2?
Yes, as of June 10, 2022, we’ve performed BLAST analysis of available whole genome sequences, and the assay’s ability to detect all current WHO variants of concern, including Delta and Omicron BA.1, BA.2, BA.3, BA.4, and BA.5 sublineages is unaffected.
The SARS-CoV-2 virus is constantly mutating as it spreads globally. We monitor sequences published to scientific databases such as GISAID on a regular basis and will update this FAQ as appropriate.
How often should I conduct COVID surveillance testing?
Most customers test twice a week. Testing at least 2 times per week allows you to detect COVID-19 in its earliest stages of infection with minimal cost and disruption to your school or workplace. As test results are representative of only the specific time of test, testing frequency is an important consideration for detecting infection. A sporadic testing regimen could lead to undetected infections in a population. Even testing once a week allows you to detect and slow the spread of COVID compared to no testing effort at all.How do RT-qPCR and rapid antigen tests compare?
RT-qPCR, or reverse-transcription quantitative polymerase chain reaction, provides accurate, quantitative results within hours. It is the current gold standard method to test for SARS-CoV-2 infection due to it’s high sensitivity. RT-qPCR can detect a miniscule amount of RNA, specific to the pathogen, and make exponential copies of it until there are over one billion copies of that particular RNA segment. This is why RT-qPCR is able to detect an infection at earlier stages, even when viral loads are low. Chai’s COVID Surveillance solution can detect an infection with as little as 5.8 viral copies per microliter of saliva.
Rapid antigen tests can provide results faster than RT-qPCR but at the expense of accuracy and sensitivity. Rapid antigen tests detect proteins on the surface of the virus, yielding qualitative results that heavily depend on higher viral loads for detection. Compared to RT-qPCR, antigen tests need a sample to contain thousands, or even tens of thousands, of viral particles per microliter to produce a positive result. In cases where the sample has low amounts of virus, rapid antigen tests may be unable to detect a COVID infection and give a false-negative result.
Multiple studies, including guidance by the CDC, concur that the sensitivity of rapid antigen tests are significantly lower than RT-qPCR. One study found that rapid antigen tests detected between 11.1% – 45.7% of RT-qPCR-positive samples from COVID-19 patients. Due to the lower sensitivity of antigen tests, the FDA and CDC recommend confirming negative results with a RT-qPCR test.
What are the benefits of saliva over swabs for surveillance testing?
Due to the minimally invasive and painless nature of saliva collection, saliva is more conducive for routine surveillance testing. Saliva is self-collected and eliminates the need to train specialized healthcare personnel on the collection process, making it easy to collect saliva on a regular basis.
Studies have also found that saliva is as sensitive if not more sensitive than nasopharyngeal swabs in detecting COVID-19. In addition, the complicated sampling procedure of nasopharyngeal swabbing increases the risk of variable and unreliable results.
Can this kit be used to test environmental surfaces?
No, this kit is intended to detect COVID-19 in a human population. However, our Coronavirus Environmental Surface Test Kit does detect SARS-CoV-2 on high-touch environmental surfaces, such as door handles, tables, shopping carts, and other hard surfaces, using the same Open qPCR technology as our surveillance testing solution. The environmental test kit can be employed as a complementary solution alongside the Coronavirus surveillance test kit to expand your testing efforts in cases where obtaining a saliva sample from every individual is not practical.
Surface swabbing is especially useful in facilities open to the public, such as hotel rooms, airplanes, and gyms. An example of using both solutions is in a restaurant setting, where the Coronavirus surveillance test kit could be used to routinely monitor workplace personnel, while environmental surface testing detects COVID-19 on high-touch surfaces in areas that are frequently exposed to visitors or customers.
Are the test kits compatible with instruments other than Open qPCR?
Yes, the test kits will work with any Real-Time PCR instrument compatible with the FAM and HEX fluorophores.Do you ship internationally?
Yes. We have distributors in most major regions. If we do not have a specific distributor in your country, we will ship and service the kits and instruments directly. Contact us at sales@chaibio.com to help you find the best way to purchase kits and instruments.How was the COVID-19 Surveillance Test Kit validated?
The test kit was validated for its sensitivity, specificity, endogenous interference and cross-reactivity, and clinical performance as detailed in the Validation Report.Why is the dual channel Open qPCR required?
A dual channel Open qPCR is required because the COVID-19 Surveillance Test Kit detects two targets, SARS-CoV-2 and the endogenous control. The SARS-CoV-2 target is detected in the FAM channel, and the endogenous control is detected in the HEX channel.How stable are saliva samples?
Saliva samples are stable at ambient temperature (25 ˚C) for up to 16 hours after collection and at 2 – 8 ˚C, for up to 72 hours after collection. For longer term storage beyond 72 hours, saliva samples are stable at -70 ˚C or below.
Resources
For Research Use Only. Not for use in diagnostic procedures.